How do chillers work? This is a question we get asked quite a lot from our subscribers. So, in this article I am going to explain the science and also talk you round the main chiller components…
The Basic Refrigeration Cycle
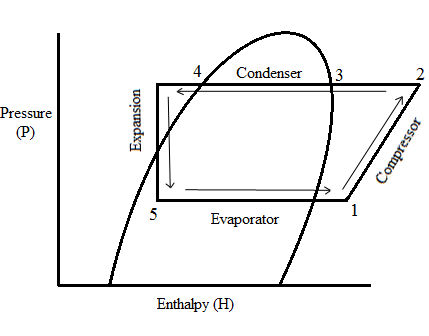
Weather it is the large centrifugal chiller like the one in the photo, or the refrigerator in your kitchen- most cooling systems work according to the basic refrigeration cycle which involves vapour compression. Two scientific principals are at work: latent heat and the pressure temperature relationship. Four chiller components are needed: evaporator, compressor, condenser and expansion valve.
Sensible heat
Heat energy that can be sensed is called sensible heat. Imagine a pan of water with a flame under it and a thermometer resting in it. You are watching the flame licking up the bottom of the pan and you are also watching the thermometer going up, obvious- right? The heat energy from the flame going into the pan is sensible heat.
Example
Now imagine if we pumped a liquid through a heat exchanger at 0°C with warm water being pumped through the other side of the heat exchanger. Some heat energy would exchange into the cold liquid and warm it up, for example, by 6°C. This is sensible heat.
0161 237 3727
service@maximuschillers.com
Latent Heat
Latent is the Latin word for hidden. Something less obvious happens when a pan of water gets to its boiling point of 100°C. You continue watching the flame licking up the bottom of the pan, but the temperature stops going up, strange- right? You carry on watching for a good while and the thermometer still does not go up? Then, eventually- the water boils into steam and the thermometer starts going up again. All of the heat energy that was going into the pan while the thermometer was not going up was latent heat. Latent heat was being absorbed into the water to cause it to change its state into steam. All of this hidden heat caused the liquid atoms to shake apart and become a gas.
Example
Now let’s look at the above example again, but this time including latent heat. At Step 5, a different liquid is pumped through the heat exchanger at 0°C which is also its boiling point. The amount of sensible heat, in our example, is still 6°C. Between the outside of the bubble and Step 1, there is 3°C of sensible superheat above the boiling point. Between Step 4 and Expansion, there is 3°C of sensible subcooling below the boiling point. The latent heat is inside of the bubble which, as you can see, has a considerable size. We measure sensible and latent heat energy using kJ/kg.
The Pressure Temperature Relationship
How we produce the above liquid at 0°C is the second scientific principle...
Higher Pressure
If you increase the pressure of a substance- the atoms are pushed together and so they get hot. The higher the pressure- the higher the temperature.
Lower Pressure
If you decrease the pressure of a substance- the atoms spread apart and so they go cool. The lower the pressure- the lower the temperature.
Refrigerant
R134a is a refrigerant that has a pressure of 1.91 barg, relative to a temperature of 0°C.
Refrigerant Saturation Point
The above temperature of 0°C is also the boiling point of R134a. This boiling point can also be called the saturation point because no more heat can be added to the liquid before it boils. The refrigerant liquid is full of heat- or saturated.
0161 237 3727
service@maximuschillers.com
How do Chillers Work with Evaporators?
This is a heat exchanger which is in between two other chiller components: the expansion valve and the compressor. The expansion valve is a restriction in the chiller system and so there is a pressure drop into the evaporator. The compressor sucks from the evaporator and so maintains this pressure drop. This is the pressure temperature relationship: the lower the pressure- the lower the temperature. Liquid refrigerant flows through the evaporator at 1.91 barg and 0°C. It boils off absorbing latent heat, then it superheats, in our example, by 3°C above its saturation point. The evaporator has absorbed heat energy from the water on the other side of the heat exchanger.
How do Chillers Work with Compressors?
A bicycle pump is a compressor- notice how it gets hot when you inflate a tyre. This is the pressure temperature relationship: the higher the pressure- the higher the temperature. Therefore, the compressor also adds heat energy into the system. The refrigerant is sucked into the compressor from the evaporator as a cold, low pressure gas at 1.91 barg. It is then compressed into a hot, high pressure gas which is discharged from the compressor at 8 barg.
How do Chillers Work with Condensers?
The hot, high pressure gas being discharged from the compressor is cooled down with fans which suck air through the fins of this heat exchanger. The gas goes through its latent heat phase again, but this time condensing from a gas and into a liquid. It is then subcooled, in our example, by 3°C below its saturation point into a hot, high pressure liquid. The heat energy absorbed in the evaporator and the heat energy added to the system by the compressor is rejected into the surrounding air.
How do Chillers Work with Expansion Valves?
The hot, high pressure liquid at 8 barg arrives from the condenser at the inlet of the expansion valve. The expansion valve could be seen as being a tap which is partially closed. This restriction causes the refrigerant to back up behind the expansion valve inlet. The refrigerant that gets through the valve and into the evaporator expands into a cold, low pressure liquid/ vapour mix. The vapour is called ‘flash gas’ and is as a result of the refrigerant expanding. Vapour is another word for a gas.
How do Chillers Work with Set Point?
This basic refrigeration cycle continues until the setpoint is achieved and the controller stops the compressor. After some time, the water warms up by a couple of degrees and the controller starts the compressor back up.
Related Articles:
Shell & Tube Chiller Evaporator Maintenance
Chilled Water System EEV Service
How do Chillers Work? Expansion Valves
How do Chillers Work? Compressors
Hit the Tags below to navigate your way to our extensive library of further reading on this subject.
Read more about vapour compression refrigeration on Wikipedia

